The difference between fluorescence and luminescence

Glowing bodies emit light. We know that from the sun, campfires or from the light bulbs that were used in the past and can still be found in the oven today, for example. The basic principle behind it is always the same: These bodies are so hot that the thermal radiation they emit, an example of electromagnetic radiation, is also in the range of visible light. The rule here is that the hotter the body, the more the heat radiation shifts in the direction of blue (and at some point even ultraviolet). This can be seen very nicely, for example, with the heating wire in a toaster, which radiates from invisible (but we can already feel the infrared radiation with our hands) to dark red to yellowish as the temperature increases: The heat radiation becomes shorter-wave until the moment of maximum heat is reached.
Luminescence, on the other hand, describes what is known as cold light. The fireflies are an example from nature. This light comes from a chemical reaction in the back of the animal, the luciferase-luciferin reaction. Here ATP, an energy source in our cells, is broken down. During this process, energy remains, which is released into the environment in the form of photons: the firefly glows without any heat radiation, thanks to bioluminescence.
This reaction is also used in the hygiene sector, in the form of ATP rapid tests, with the help of which surfaces are checked for their degree of purity. In this case the ATP comes from cells that were present on the surface, luciferase and luciferin are artificially produced and added. The more the sample then glows, the more germs there were on the tested surface.
Electro-luminescence: This is how LEDs work
Luminescence can also be produced electrically under certain conditions. This is, among other things, the functional principle behind light emitting diodes. LEDs are essentially semiconductor crystals that consist of two materials that are doped differently: one part has more negative charge carriers, another more positive. Doping means that small amounts of another substance have been introduced into the semiconductor material, so that either more electrons (n-doping) or holes, i.e. too few electrons (p-doping) are present. The semiconductor material silicon, for example, has 4 outer electrons (so-called valence electrons). If it is contaminated with a pinch of phosphorus, which has 5 outer electrons, then an n-doped layer can be produced in this way.
These additional electrons are stimulated to move by an applied current. When they return to their original state, they then give off the excess energy in the form of photons, i.e. light. One of the discoverers of this principle, Oleg Vladimirovich Losev, had described the effect in his publication at the time as cold light, because it was obviously not caused by the heat radiation of a glowing material.
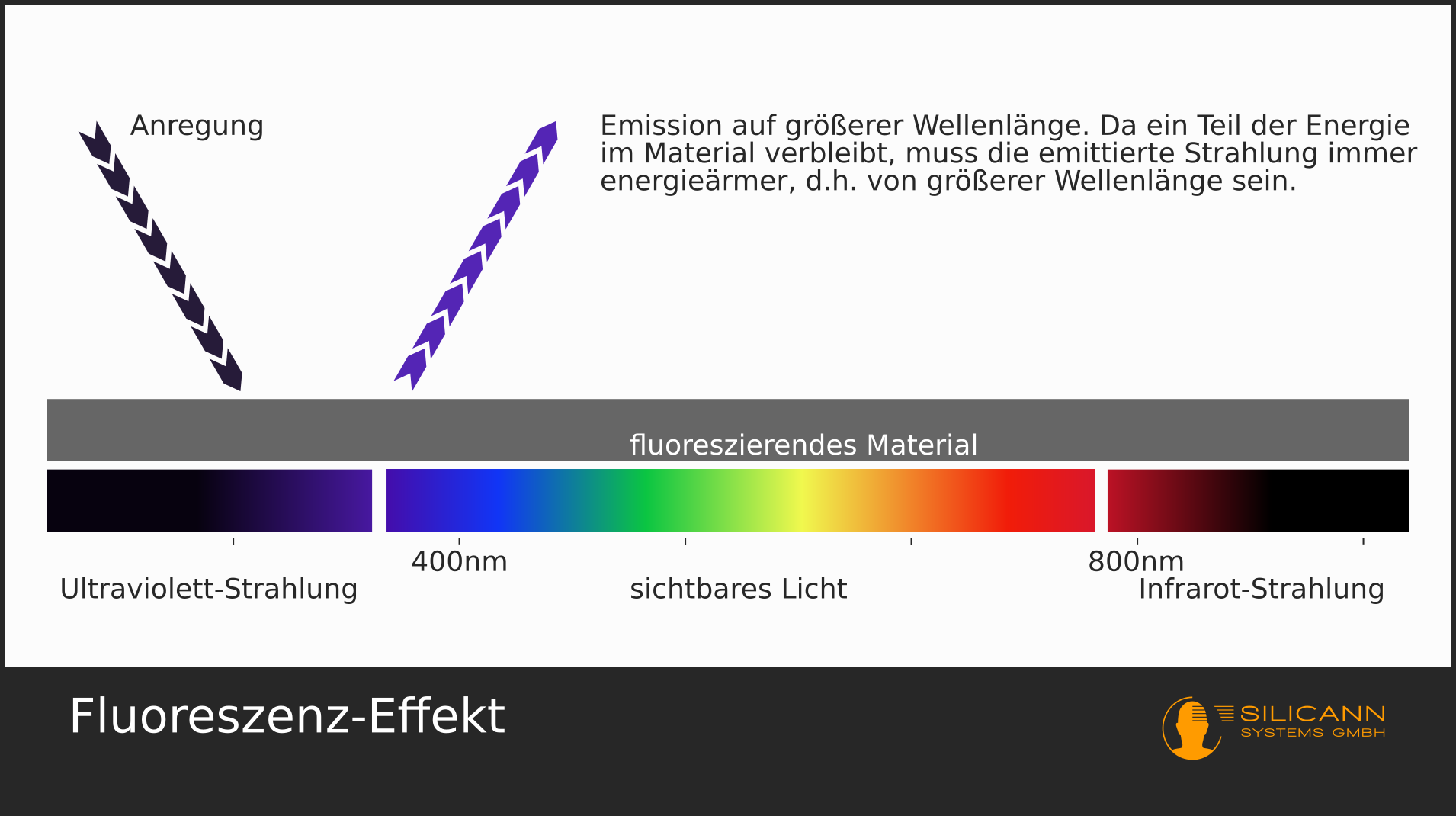
Fluorescence
Fluorescence works in a very similar way, here the electrons are not excited electrically. Instead, substances are excited with light of a very specific wavelength, i.e. the photons hit the particles in the substance and these particles are set in vibration. Then they return to their basic state and give off the excess energy in the form of photons. Since the oscillation has consumed part of the energy, the emitted photons are lower in energy, i.e. they have a longer wavelength than the light with which the substance was originally irradiated. For example, the small luminous markings in euro banknotes are excited with UV light ("black light", with a wavelength of approx. 365 nm) and then fluoresce back in different colors of visible light (at approx. 450-700 nm).
Does that sound almost identical to the description above? It is - fluorescence is another example of luminescence. Here you can find more information about the fields of application of fluorescence.



